Study Description
The Mexican Health and Aging Study (MHAS) is a national longitudinal
study of adults 50 years and older in Mexico.
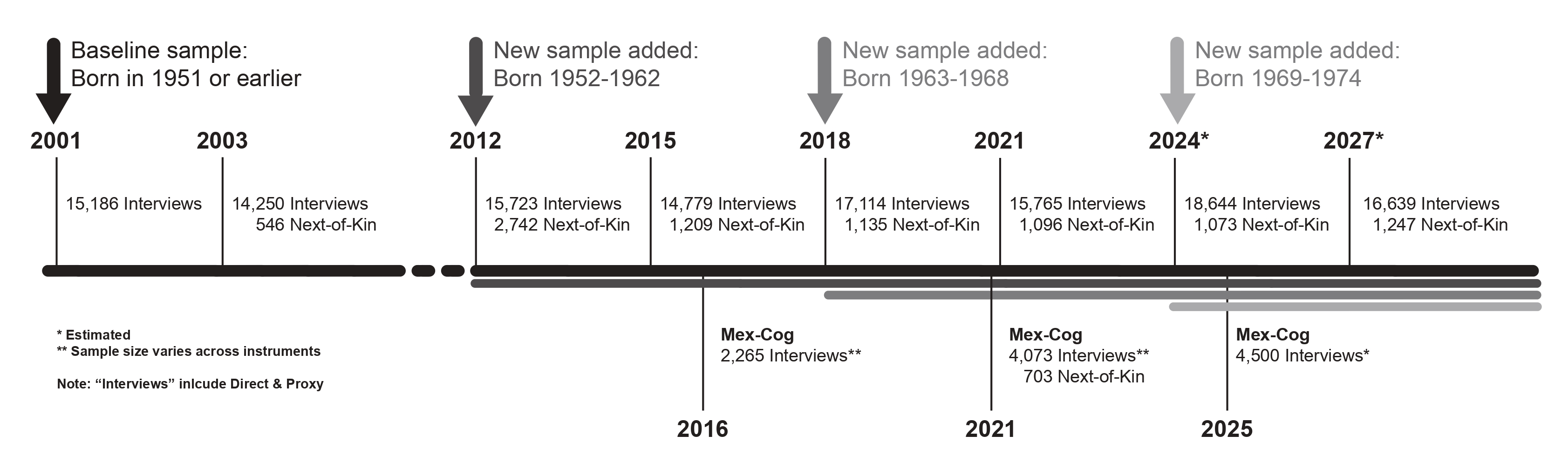
The baseline survey, with national and urban/rural representation of adults born in 1951 or earlier, was conducted in 2001 with
follow-up interviews in 2003, 2012, 2015, 2018, 2021 and 2024. A new sample of adults born between 1952-1962 was added in 2012.
Similarly, in 2018 a new cohort of adults born between 1963 and 1968 was added to refresh the sample.
The study is a collaborative effort among researchers from the University of Texas Medical Branch (UTMB), the Instituto Nacional
de Estadística y Geografía (INEGI, Mexico), the Instituto Nacional de Geriatría (INGER, Mexico), the Instituto Nacional de Salud
Pública (INSP, Mexico), Columbia University, and University of California Los Angeles (UCLA). The MHAS is partly supported by the
National Institutes of Health/National Institute on Aging (R01AG018016, R Wong, PI) in the United States and the Instituto Nacional
de Estadística y Geografía (INEGI) in Mexico.
2001 & 2003
The survey has national and urban/rural representation. The baseline survey, in 2001, included a nationally representative sample
of Mexicans aged 50 and over and their spouse/partners regardless of their age. A direct interview was sought with each individual
and proxy interviews were obtained when poor health or temporary absence precluded a direct interview.
The sample was distributed in all 32 states of the country in urban and rural areas. Households in the six states which account for
40% of all migrants to the U.S. were over-sampled.
All interviews were conducted in person with paper-pencil by trained full-time interviewers of the Instituto Nacional de Estadística y Geografia (INEGI)
of Mexico.
A follow-up interview with surviving respondents was conducted in 2003. The follow-up survey protocol included: a follow-up interview with all surviving
respondents, a next-of-kin interview about deceased respondents; a complete baseline interview for new spouse/partners; and a proxy interview for
respondents unable to complete their own interview because of illness or temporary absence.
2012 & 2015
A third follow-up survey that was conducted in 2012. For the 2012 wave, interviews were conducted for every person who was part of the panel in 2003 and
their new spouse/partner, if applicable, and a new sample of persons born between 1952 and 1962. Interviews were conducted person-to-person using CAPI
(Computer-Assisted Personal Interviewing) by INEGI.
A fourth round of the longitudinal study was completed in 2015.
Similar to 2003, the study protocol included a follow-up interview with all the surviving respondents that had completed at least one interview since 2001.
In addition, the protocol included those from the new sample added in 2012 that could not be contacted in 2012. Interviews were conducted person-to-person,
using CAPI by INEGI. Direct interviews were sought with all the informants, but proxy interviews were completed for those unable to complete their own interview.
A next-of-kin interview was completed for those who were part of the panel but died between surveys.
2018 & 2021
A fifth follow-up survey that was conducted in 2018 using the same protocols established since the second wave. Also, similar to the third wave, a new
sample of persons born between 1963 and 1968 was added to the sample. A fifth round of the longitufinal study was completed in 2021.
2024
A seventh follow-up survey that was conducted in 2024 using the same protocols established since the second wave. A new sample of persons born between 1969 and 1974 was added to the sample.
An eighth round of the longitudinal study is planned for 2027.
Our Timeline
Survey Contents
The MHAS content includes:
- Health in multiple domains (self-report of global health, chronic conditions, symptom reports, functionality, depression, cognition).
- Socioeconomic conditions (current and in childhood), work history, health insurance, health expenditures.
- Family background (family structure, transfer behaviors, care arrangements, health and migration histories of respondents, parents and children), children (regardless of place of residence) and household residents.
- Income, assets, pension history, current housing, and quality of built environment.
- Major events over the last 10 years (only in 2012): change of residence, major health event, natural disaster, or crime event.
- Time use and psychosocial aspects (locus of control, life satisfaction, loneliness, and conscientiousness).
- For deceased study participants, last year of life: major events, use of health services, care receive, and functionality.
- Sub-sample for biomarkers, anthropometrics, and performance measures (only in 2012).
Ancillary Studies
- In 2001 and 2003, a sub-sample was selected to obtain anthropometric measures.
- In 2012, a sub-sample was selected to obtain objective markers such as blood sample and anthropometric measures.
- In 2016, a sub-sample of the 2015 survey was selected to complete an in-depth cognitive assessment. The Cognitive Aging
Ancillary Study (Mex-Cog) uses a harmonized cognitive assessment protocol (HCAP). Adapted versions of the HCAP have been
used to assess cognition in other subsamples within ongoing population-based longitudinal studies of aging around the
world.
- In 2018, two sub-samples were selected for two separate ancillary studies. All individuals aged 60 or older, who complete a direct interview were asked to participate in the collection of saliva
to complete a study on genetics and dementia. The second sub-sample included the sample in 5 states of Mexico. The states were selected to represent the national
sample. All individuals were asked to participate in the collection of hair sample to study environmental exposure.
Publications of interest:
Wong R, Michaels-Obregon A, Palloni A. Cohort Profile: The Mexican Health and Aging Study (MHAS).
Int J Epidemiol. 2017 Apr 1;46(2):e2. doi: 10.1093/ije/dyu263. PMID: 25626437; PMCID:
PMC5837398.
Mejia-Arango S, Nevarez R, Michaels-Obregon A, Trejo-Valdivia B, Mendoza-Alvarado LR, Sosa-Ortiz AL,
Martinez-Ruiz A, Wong R. The Mexican Cognitive Aging Ancillary Study (Mex-Cog): Study Design and
Methods. Arch Gerontol Geriatr. 2020 Jul 27;91:104210. doi: 10.1016/j.archger.2020.104210. Epub ahead
of print. PMID: 32781379; PMCID:
PMC7854788.